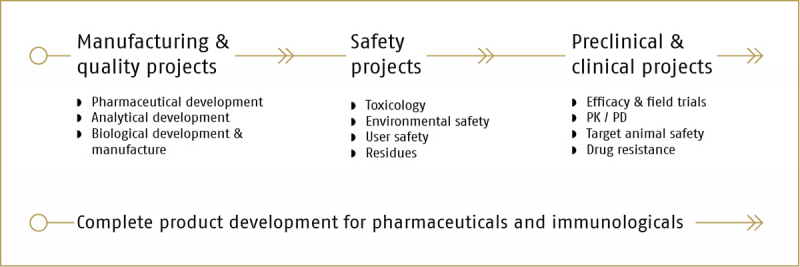
Whether you are looking to carry out a single study during the development of your product or you are looking for a partner to whom you can outsource the complete drug development process, Cyton has the expertise and experience to support you.
The drug development process is not cheap and Cyton understands the pressures in what is an increasingly competitive industry. With Cyton as your partner, you can be sure that the data you generate for your product will be fully compliant with all regulatory requirements, without you incurring unnecessary expense.
If you are not looking to outsource the complete drug development process for your products, Cyton can provide the following specific services:
- Gap analysis and data audit for your product identifying supported claims and additional data requirements
- Conducting literature searches and evaluation of published data to support your product
- Preparation of development plans with estimates of costs and timelines
- Study design and reporting in compliance with all relevant guidelines
- Independent selection of Contract Research Organisations / Contract Manufacturing Organisations to conduct studies
- Biological development and manufacture: strategic advice and support including process development and validation, QC test development and validation, technology transfer
Find out more about our technical teams in the links provided in the panel to the right.